How To Draw Face Centred Cubic Packing
What Does Face-Centered Cubic (FCC) Mean?
Confront-centered cubic (FCC or cF) is the name given to a type of atom arrangement institute in nature. A face-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube has a fraction of an atom with 6 boosted full atoms positioned at the center of each cube face.
The atoms at the corner of the cube are shared with eight other unit of measurement cells. Every bit such, each corner atom represents 1-eighth of an atom.
The atoms at each face of the unit jail cell are shared with adjacent unit cells; therefore, each confront atom represents half of an cantlet.
Using this concept, the total number of atoms in the FCC unit of measurement cell structure is 4; six halves at each of the faces, plus eight one-eighth atoms at the corners.
(1/ii atoms x 6 faces) + (i/viii atoms x 8 corners) = 4 atoms
The atoms in the FCC unit of measurement cell arrangement are packed closer than other cell arrangements (such every bit the body-centered cubic (BCC), which is the simple unit cell arrangement). Due to their packing arrangement, FCC metals are typically softer and more ductile than their BCC counterparts are, which may exist an of import gene when selecting materials for a given application.
Face-centered cubic may also be known as cubic close-packed (ccp).
Corrosionpedia Explains Confront-Centered Cubic (FCC)
Metallic atoms naturally pack themselves in a close arrangement to class the strongest metallic bond possible. In nature, several packing arrangements are possible, including the face-centered cubic organisation.
One of the defining features of FCC is that the atoms are packed as close together as theoretically possible. The atoms from one layer nest themselves snugly into the empty space of each adjacent layer.
This close packing system is quantified in FCC's packing density.
Some metals that possess this crystalline construction include aluminum, golden, atomic number 82, platinum, iridium and silver.
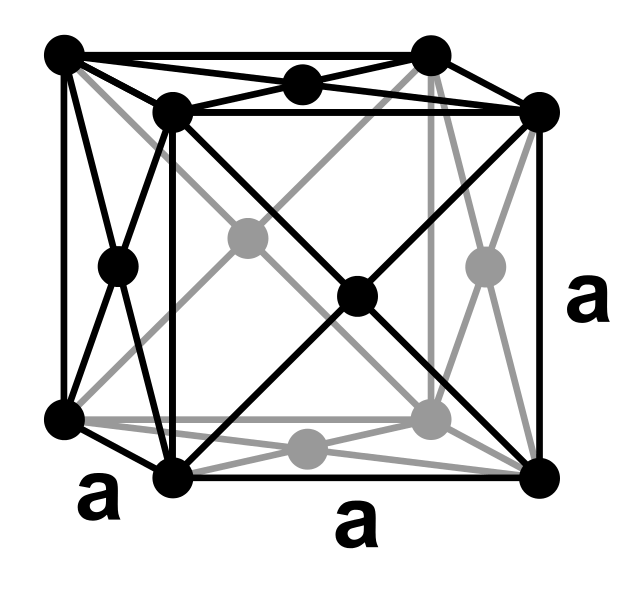
Figure i. Analogy of a Face-Centered Cubic Structure. (Source: Wikimedia Commons)
Packing Density / Diminutive Packing Factor (APF)
The packing density, also known equally the atomic packing gene (APF), is essentially the fraction of the volume of atoms that occupy a crystal structure.
The APF of an FCC structure is equal to the volume of the atoms in the unit of measurement cell divided past the volume of the unit cell.
Therefore:
APFFCC = Vatoms/Vunit cell
Because each atom is represented as a sphere and the unit of measurement jail cell is a cube:
APFFCC = n·Vsphere/Vcube (where n = number of atoms calculated previously)
APFFCC = (4 x iv/3πr3)/a3
To further break this downwards, nosotros tin limited "a" in term of "r".
Using Pythagoras' Theorem: aii + a2 = (4r)2
Solving for "a" we get, a = 2√ii.r
Taking this result for "a" and putting information technology back into the formula for APF, the equation becomes:
APFFCC = (4 ten 4/3πrthree)/ (ii√ii.r)three
Cancelling common terms, nosotros get APFFCC = 0.74. In other words, 74% of the book of the unit cell is occupied by atoms. This value is referred to as shut-packed because information technology is not possible to pack the atoms whatever closer to achieve a college APF.
Source: https://www.corrosionpedia.com/definition/1592/face-centered-cubic-fcc
Posted by: stormplacrour.blogspot.com


0 Response to "How To Draw Face Centred Cubic Packing"
Post a Comment